Voltage hysteresis or Passivation film in lithium thionyl chloride batteries/LiSOCL battery
Voltage hysteresis or Passivation film in lithium thionyl chloride batteries/LiSOCL battery
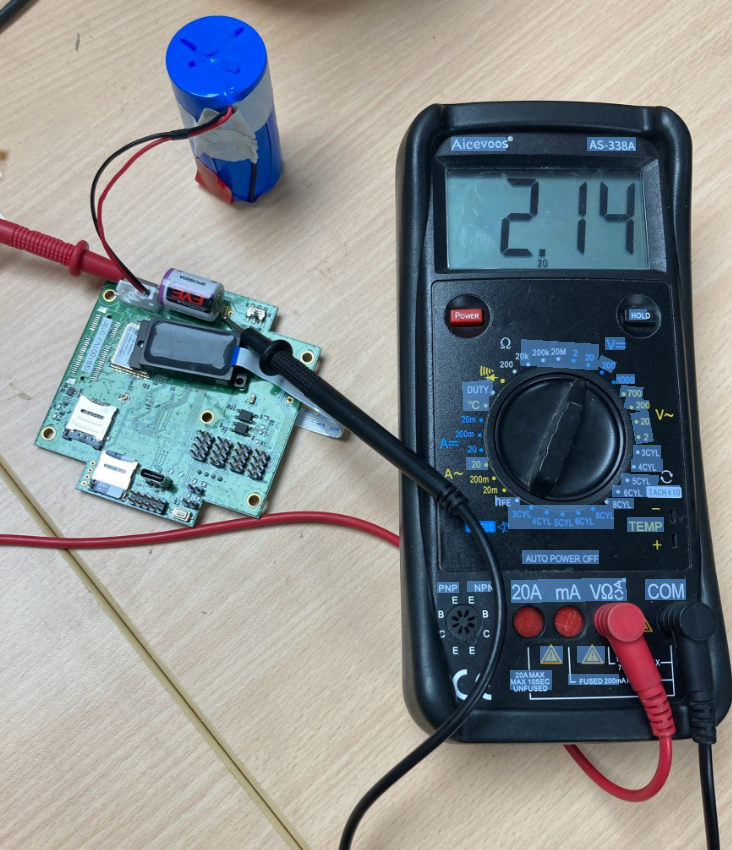
The formation of passivation film in lithium thionyl chloride batteries, also known as oxide film, primarily occurs during the chemical reactions within the LiSOCL2 battery. Below is a detailed mechanism of how the passivation film forms in lithium thionyl chloride batteries:
Formation Process
When the lithium metal anode in a lithium thionyl chloride battery reacts chemically with thionyl chloride in the electrolyte, lithium chloride (LiCl) is produced as an oxidation product. This lithium chloride gradually deposits on the surface of the lithium metal anode, forming a layer of passivation film.
Chemical Equation
The overall reaction in a lithium thionyl chloride battery can be expressed as:
2SOCl2 + 4Li → 4LiCl + SO2 + S
In this reaction, lithium metal (Li) reacts with thionyl chloride (SOCl2) to produce lithium chloride (LiCl), sulfur dioxide (SO2), and sulfur (S). Among these, lithium chloride is the main component that forms the passivation film.
Role of Passivation Film
Protective Effect: The passivation film prevents continuous rapid reactions between the lithium metal anode and the electrolyte, thereby protecting the anode from excessive consumption.
Impact on LiSOCL2 Battery Performance: The presence of the passivation film affects the internal resistance and self-discharge rate of the battery. As the passivation film gradually thickens, the internal resistance of the battery may change, and the self-discharge rate will also be affected.
Voltage Lag Phenomenon: After prolonged discharge at a small current or storage at rest, lithium thionyl chloride batteries may exhibit a voltage lag when discharged at a high current. This is primarily caused by changes in the rate of chemical reactions within the battery due to the presence of the passivation film.
Influencing Factors
Storage Conditions: The storage temperature and duration of the battery affect the formation rate and thickness of the passivation film. High temperatures accelerate the passivation process, while low temperatures may slow down the reaction rate.
Battery Design: The structure and material selection of the battery also influence the formation of the passivation film. For example, the composition and concentration of the electrolyte, the type and morphology of the anode material, etc., may all have an impact on the formation of the passivation film.
In summary, the passivation film in lithium thionyl chloride batteries is formed during the chemical reactions within the LiSOCL2 battery and is primarily composed of products such as lithium chloride. It has a significant impact on the performance and storage characteristics of the LiSOCL2 battery and therefore needs to be considered and controlled in practical applications.





