Passivation of Lithium Batteries: Effects in High-Temperature LiSOCL2 batteries
Passivation of Lithium Batteries: Effects in High-Temperature LiSOCL2 batteries
Introduction
Lithium batteries have become ubiquitous in modern society, powering everything from portable electronics to electric vehicles. One of the key characteristics that enable their widespread use is the passivation layer formed on the lithium anode. This layer, while crucial for battery function, can also present challenges, particularly in high-temperature applications. This article delves into the phenomenon of passivation in lithium batteries, focusing on its implications for high-temperature batteries, specifically LiSOCl2 batteries and high-temperature LiSOCl2 batteries.
Understanding Passivation in Lithium Batteries
Passivation is a natural phenomenon that occurs in lithium batteries, where a very thin, high-resistant, self-assembled layer forms on the surface of the lithium anode. This layer is the result of a chemical reaction between the battery's electrolyte and the lithium anode. The passivation layer, often referred to as the solid-electrolyte interphase (SEI), plays a critical role in the battery's performance and longevity.
Without the passivation layer, lithium batteries would be highly unstable. The lithium anode would react rapidly with the electrolyte, leading to rapid discharge and degradation of the battery. The passivation layer acts as a barrier, preventing further reaction and stabilizing the anode surface. This stabilization allows the battery to maintain a low self-discharge rate and a long shelf life, making it suitable for various applications.
The Role of the Passivation Layer
The passivation layer's primary function is to protect the lithium anode from further reaction with the electrolyte. It achieves this by forming a high-resistant barrier that limits the diffusion of lithium ions and electrons to the electrolyte. While this barrier is essential for battery stability, it can also introduce challenges, particularly in terms of battery performance.
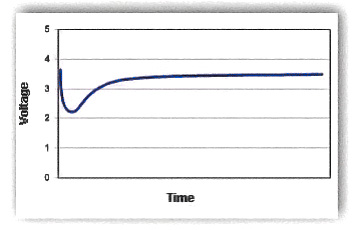
One of the most noticeable effects of the passivation layer is voltage delay. When a load is applied to the battery, the high resistance of the passivation layer causes the voltage to drop rapidly. As the battery discharges, the passivation layer is gradually removed, lowering the internal resistance and allowing the voltage to stabilize. However, if the load increases after the voltage stabilizes, the voltage may drop again until the passivation layer is fully removed.
Implications for High-Temperature Batteries
High-temperature batteries, such as LiSOCl2 batteries, are designed to operate in environments where temperatures can exceed the normal operating range of conventional lithium batteries. These batteries are crucial for applications in aerospace, automotive, and industrial sectors, where exposure to extreme temperatures is common.
In high-temperature batteries, the passivation layer plays a dual role. On one hand, it protects the lithium anode from degradation at elevated temperatures. On the other hand, the increased temperature can accelerate the formation and growth of the passivation layer, potentially leading to increased internal resistance and reduced battery performance.
LiSOCl2 Batteries: A Case Study
LiSOCl2 batteries are a type of lithium battery that uses lithium thionyl chloride (LiSOCl2) as the electrolyte. These batteries are known for their high energy density, long shelf life, and ability to operate in a wide temperature range, including high temperatures. However, the passivation phenomenon in LiSOCl2 batteries can be particularly pronounced due to the nature of the electrolyte.
In LiSOCl2 batteries, the passivation layer forms as a result of the reaction between the LiSOCl2 electrolyte and the lithium anode. The layer primarily consists of lithium chloride (LiCl) and other decomposition products of the electrolyte. While this layer protects the anode, it can also introduce significant voltage delay and reduce the battery's discharge efficiency, especially at high temperatures.
High-Temperature LiSOCl2 Batteries: Challenges and Solutions
High-temperature LiSOCl2 batteries are designed to operate in environments where temperatures can exceed 85°C. In such conditions, the passivation layer can become even more pronounced, leading to increased internal resistance and reduced battery performance. To mitigate these effects, several strategies can be employed:
Electrolyte Additives: Adding specific additives to the LiSOCl2 electrolyte can help modify the composition and properties of the passivation layer. These additives can promote the formation of a more stable and conductive passivation layer, reducing voltage delay and improving discharge efficiency.
Anode Coatings: Applying a protective coating to the lithium anode can help prevent direct contact between the anode and the electrolyte, reducing the formation of the passivation layer. This can improve the battery's performance and longevity, particularly in high-temperature applications.
Thermal Management: Implementing effective thermal management strategies, such as using heat sinks or phase-change materials, can help regulate the battery's operating temperature. By keeping the temperature within the optimal range, the formation and growth of the passivation layer can be minimized, improving the battery's performance and longevity.
Battery Design: Optimizing the battery's design, such as the electrode configuration and separator material, can also help mitigate the effects of passivation. For example, using a separator with high ionic conductivity can help reduce the internal resistance of the battery, compensating for the increased resistance introduced by the passivation layer.
Conclusion
Passivation is a critical phenomenon in lithium batteries, playing a crucial role in their stability and longevity. However, it can also introduce challenges, particularly in high-temperature applications. By understanding the mechanisms behind passivation and employing appropriate mitigation strategies, the performance and longevity of high-temperature lithium batteries, such as LiSOCl2 batteries, can be significantly improved.
As technology continues to advance, further research into the passivation phenomenon and the development of novel mitigation strategies will be crucial for enabling the widespread adoption of high-temperature lithium batteries in various applications. By addressing the challenges associated with passivation, we can unlock the full potential of these batteries and pave the way for a more sustainable and efficient energy future.





